
Research on Inspecting the outer packing film of cigarette box by Raman Spectroscopy
Research on the Examination of Cigarette Pack Outer Wrapping Films Using Raman Spectroscopy
Lu Yifan¹, Jiang Hong¹, Ma Teng¹, Su Hang¹, Zhang Liwen²
(1. People’s Public Security University of China, Beijing 100038, China
2. Beijing Zhuoli Hanguang Instruments Co., Ltd., Beijing 101102, China)
Excerpted from Shanghai Plastics, Issue 04, 2017
1. Introduction
With the continuous increase in the smoking population, the presence of cigarette-related physical evidence at crime scenes has gradually grown. Analyzing cigarette brands and their sources has become particularly important for narrowing investigative scopes and effectively combating criminal activity. At present, significant research progress has been made on cigarette butts, ash, tobacco, outer packaging films, and inner liners of cigarette packs.
The outer wrapping film of cigarette packs refers to the film covering individual cigarette boxes and cartons. Over 85% of cigarette packs worldwide utilize transparent packaging materials. These films possess excellent barrier properties, transparency, heat sealability, mechanical processability, and low oxygen and moisture permeability. They effectively prevent moisture absorption and mildew in cigarettes while minimizing the loss of aroma, thereby offering both protective and aromatic retention functions [1].
Currently, forensic science methods used for analyzing cigarette pack outer wrapping films include infrared spectroscopy, small-angle X-ray scattering, differential scanning calorimetry, and X-ray fluorescence spectroscopy [4–9]. Raman spectroscopy, with its advantages of being non-destructive, rapid, accurate, and requiring no sample pretreatment, has been widely applied in fields such as agriculture, medicine, and chemistry. In this study, Raman spectroscopy was used to examine and differentiate outer wrapping films of cigarette packs, yielding promising results.
2. Experiment
2.1 Instruments and Conditions
Instrument: Finder Vista Micro Confocal Laser Raman Spectrometer (Beijing Zhuoli Hanguang Instruments Co., Ltd.)
Conditions:
-
Laser wavelength: 532 nm
-
Laser power: 10 mW
-
Spectral range: 3123 cm⁻¹ to 439 cm⁻¹
-
Acquisition time: 3 seconds
-
Accumulation: 5 scans
2.2 Samples
A total of 48 outer wrapping film samples from different brands and series of cigarette packs were tested, including Huagui Taishan, Peony, Hongtashan, and Zhongnanhai filtered cigarettes.
2.3 Method
Samples were placed on the microscope stage with the inner side (the surface in contact with the cigarette box) facing up. Tests were conducted in dark conditions. Each sample was measured three times, and the average values were taken.
3. Analysis of Results
3.1 Classification Based on Raman Spectra
First, the Raman spectra of the samples were compared with the standard spectrum of polypropylene (see Figure 3) [10].
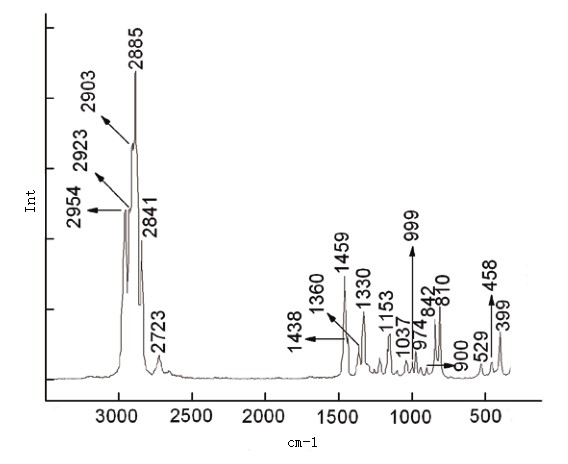
Figure 3. Standard Raman spectrum of polypropylene
Polypropylene is characterized by peaks at 2954, 2923, 2903, 2885, 2841, 2723, 1459, 1438, 1360, 1330, 1167, 1147, 973, 842, 810, 529, 456, and 399 cm⁻¹.
-
Peaks in the 3000–2600 cm⁻¹ range mainly correspond to stretching vibrations of C-H bonds in CH₃ and CH₂ groups.
-
Peaks in the 1500–800 cm⁻¹ range represent C-C bond stretching and C-H bending vibrations.
-
Peaks in the 550–300 cm⁻¹ range correspond to skeletal waggling vibrations of the polypropylene carbon chain [11].
Through comparative analysis, all 48 samples were found to be primarily composed of polypropylene. The main differences among samples were observed in the 2600–1500 cm⁻¹ range. Based on variations in spectral peaks within this range, the samples were classified into four categories:
3.1.1 Type I: Samples with Cis-Trans Isomerism of Carbon-Carbon Double Bonds
Cis-trans isomerism is a form of stereoisomerism arising because the two carbon atoms connected by a double bond cannot freely rotate around the σ bond axis. In the biaxial stretching process used in polypropylene film production, relaxation can occur during or after stretching. If relaxation has less impact than stretching on molecular deformation, polymers are prone to bond breaking during stretching. Carbon-carbon single bonds formed by polymerization may revert to double bonds under these conditions, forming cis-trans structures. These exhibit strong peaks at 2250–2150 cm⁻¹ (see Figure 4). Five samples fell into this category, mainly produced by Hongyun Honghe Tobacco Group Co., Ltd., Tianjin Cigarette Factory, and the Shandong and Henan branches of China Tobacco Industry Co., Ltd.
Figure 4. Raman spectrum of Type I, Sample #23
3.1.2 Type II: Samples with Alkynyl (C≡C) Structures
These samples exhibited strong characteristic peaks in the 2350–2250 cm⁻¹ range (see Figure 5). During the crystallization of liquid polypropylene, molecular structures tend toward stability, forming ordered crystalline arrangements that enhance optical and mechanical properties [11]. In the final heating stage, molecular instability at high temperatures can result in the formation of C≡C bonds. Seven samples belonged to this category; one was produced by Hongta Tobacco Co., Ltd., and the rest by China Tobacco Industry Co., Ltd.
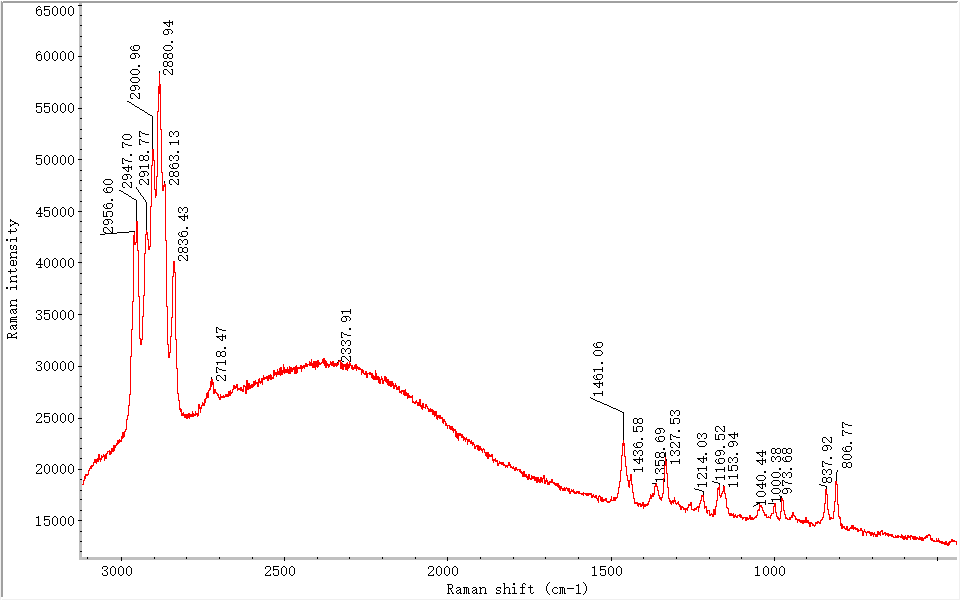
Figure 5. Raman spectrum of Type II, Sample #1
3.1.3 Type III: Mixed-Type Samples
These samples exhibited characteristic peaks in both the 2350–2250 cm⁻¹ and 2250–2150 cm⁻¹ regions (see Figure 6), indicating the presence of both C=C and C≡C structures. Differences in stretching temperatures and speeds may have led to double bond formation, while variations in final heating conditions resulted in triple bond structures.
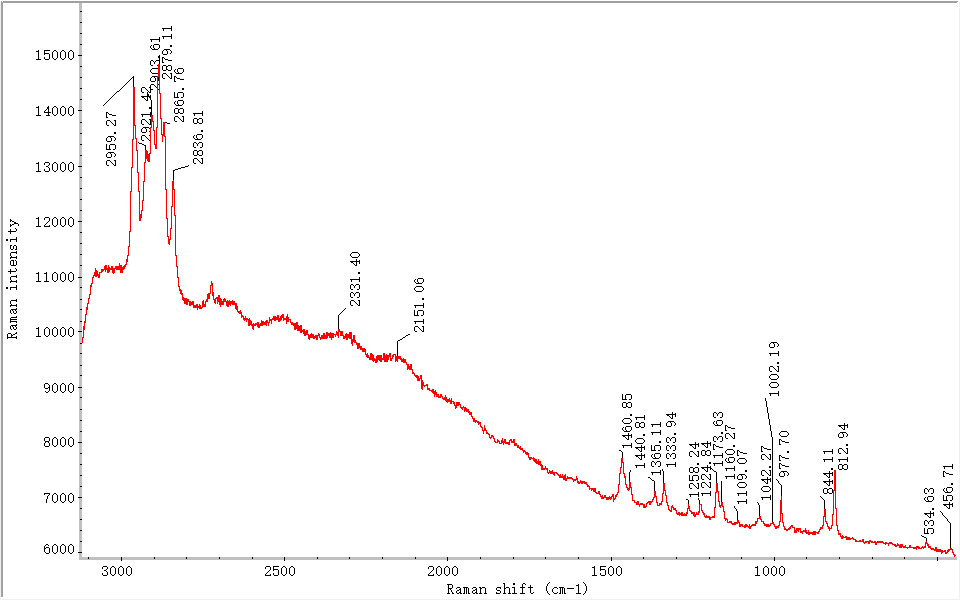
Figure 6. Raman spectrum of Type III, Sample #28
3.1.4 Type IV: Pure Samples
These spectra were highly consistent with standard polypropylene spectra, showing minimal additional carbon-carbon bond structures (see Figure 7). The high polypropylene content in these samples made them harder to distinguish. This type was the most common and sourced from a wide variety of manufacturers.

Figure 7. Raman spectrum of Type IV, Sample #2
4. Conclusion
Raman spectroscopy can effectively be used to examine cigarette pack outer wrapping films. The technique is simple, rapid, accurate, non-destructive, and requires no pretreatment. Based on Raman spectra, the samples can be classified, providing valuable support for law enforcement investigations.
References
[1] Feng Shuming. Discussion on the Application of Heat-Sealable Polyester Films (Sealable PET) in Cigarette Packaging [J]. China Packaging Industry, 2009 (Z1): 53–54.
[2] Dong Kun, Rao Zhifan, Yang Xiaoyun. Raman Spectroscopy Detection of Several Plastics [J]. Plastics Industry, 2011 (6): 67–70.
[3] Tu Zhigang, Zhang Liqiong. Development of High-Performance and Functional BOPP Films [J]. Journal of Packaging, 2012 (2): 6–12.
[4–9] (Infrared and X-ray methods in cigarette packaging analysis – summarized in the original)
[10] Chen Hesheng, Sun Yubin. FT-Raman Spectral Analysis of Several Plastics [J]. Plastics Technology, 2012 (6): 69–72.
[11] Yang Yao, Huang Zhengliang, Wang Jingdai, Jiang Binbo, et al. Raman Spectroscopy for Analyzing Properties of Propylene Copolymers [J]. Spectroscopy and Spectral Analysis, 2012 (12): 3262–3266.
[12] Su Hang, Jiang Hong, Chen Yutai, et al. Research on the Use of Raman Spectroscopy in Thermal Paper Analysis [J]. 2017 (16).
